Validated testing to eliminate contamination risks
Contamination from pharmaceutical gases is an invisible threat that can critically undermine product safety, patient health, and regulatory approval. To ensure product quality, their purity must be consistently monitored and tightly controlled. As such, verifying that gases such as nitrogen, oxygen, and carbon dioxide meet pharmacopeial standards is a vital part of quality control. But conducting pharmaceutical gas testing isn’t easy. It requires validated sampling methods, highly specialised equipment, and technicians trained and qualified to meet both security and safety challenges.
The Eurofins BioPharma Product Testing network of laboratories provides pharmaceutical gas testing with the precision, expertise, and compliance your processes demand. Our dedicated equipment, qualified methods, and specialist teams are ready to help you mitigate risks and maintain the highest quality and safety standards of your pharma product, which could be contaminated through the use of pharmaceutical gases during production.
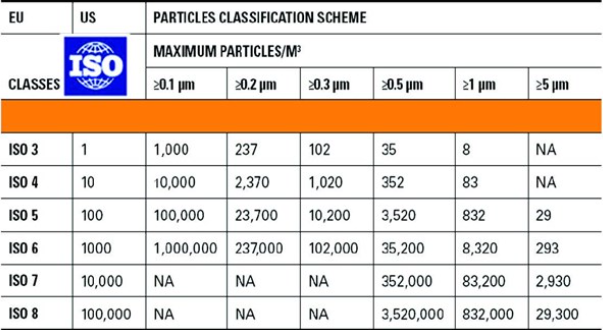
The gases that are most commonly used in the pharmaceutical industry are: nitrogen for inerting or flushing; air for flushing; oxygen for fermentation; and carbon dioxide for extraction and purification. The quality of those gases is specified in the European and U.S. pharmacopeias. Besides the pharmacopeial specifications, we also test the gases in terms of particular and microbial contamination. The contamination of the gas should not be higher than the contamination of the room where it is used. As an example, a gas used in an ISO 5 room, corresponding to the class 100, should be analysed according to the ISO 5 specification. The specifications for the particular and microbial contamination are reported in tables 1 and 2.
Our on-site sampling techniques are fully validated, designed to ensure representative gas samples. We provide detailed Certificates of Analysis and full traceability for audit readiness.
Table 1: Specification and methods according to the European Pharmacopeia
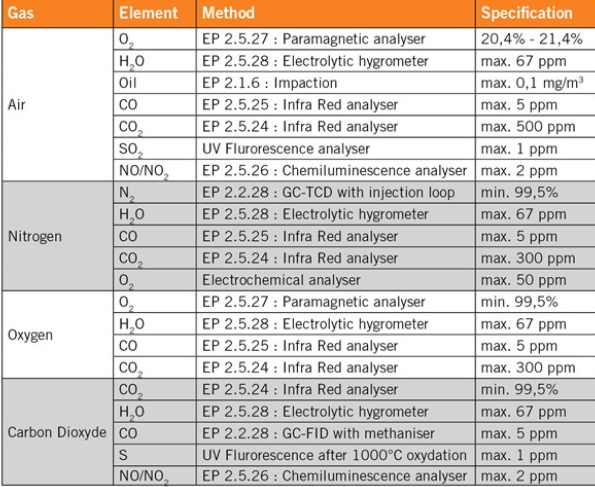
Table 2: ISO clean room standard for particles