Expert Monocyte Activation Testing for Parenteral formulations
Evolving techniques for pyrogen testing have been fully adapted by the pharmacopeias. Since 1 July 2025, many regulatory authorities require pharmaceutical companies to adopt the Monocyte Activation Test (MAT) instead of the Rabbit Pyrogen Test (RPT) for any new parenteral product . This shift, driven by regulatory, ethical, and scientific imperatives, places new demands on quality teams and manufacturing operations.
You need a validated, accurate method to test injectables that ensures patient safety, and avoids reliance on in-vivo testing. You also require a partner that will help you avoid costly infrastructure investments, navigate risk assessments, and overcome technical hurdles such as interference testing, Low Endotoxin Recovery (LER) masking effects, and Non-Endotoxin Pyrogens NEP detection.
The Eurofins BioPharma Product Testing (BPT) network of laboratories delivers comprehensive MAT services that address all of these challenges.

Eurofins BPT supports clients in aligning current pharmacopoeial guidance:`
- Ph. Eur. 2.6.30 and 2.6.40 (Europe)
- USP <151> (USA), allowing validated in vitro alternatives to RPT
- JP G4-13-190 (Japan)
- IP 8th edition (India)
- CHP Guideline 9301 (China)
Our experts continuously monitor regulatory updates to ensure that tests are performed in compliance with current requirements. We also carry out risk assessments required under European guidance when products or processes change, particularly to confirm absence of NEPs undetectable by Lumulus Amebocyte Lysate (LAL).
Technical barriers

While MAT provides semi-quantitative results and is the more ethically acceptable approach compared to RPT, it demands product-specific validation that includes:
- Interference and comparative studies
- Compatibility checks for products that affect detection systems
Alongside customised demasking strategies for Low Endotoxin Recovery (LER)
Our specialists, in collaboration with our regularly trained laboratory team experienced in handling a wide variety of sample types, will select the most suitable MAT kit for your needs—eliminating the need to in-source MAT systems or invest in training in-house teams.
End-to-end support

- Regulatory consultation and MAT kit selection
- Tailored study designs
- Access to multimode plate readers compatible with most MAT kits
- Safe handling and storage of primary cells and cell lines
- 21 CFR Part 11–compliant software to ensure that you meet data integrity requirements
MAT services

- Batch release testing
- NEP detection in late-stage development
- LER studies - required for biological drug products (protein-based products, vaccines and gene therapies) containing a combination of a surfactant (e.g., polysorbate) and a chelator (e.g., EDTA, citrate). LER studies are also required by the FDA for Biologics License Applications (BLAs)
- Demasking studies for biologics
- Alternate endotoxin testing method when LAL or recombinant Factor C (rFC) are insufficient
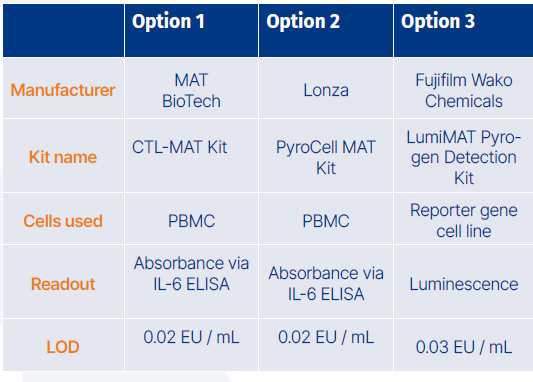
Established MAT kits

What Eurofins BPT offers you is pyrogen testing without compromise: ethical, accurate, and enabling you to meet compliance expectations.