Our solutions
Our services
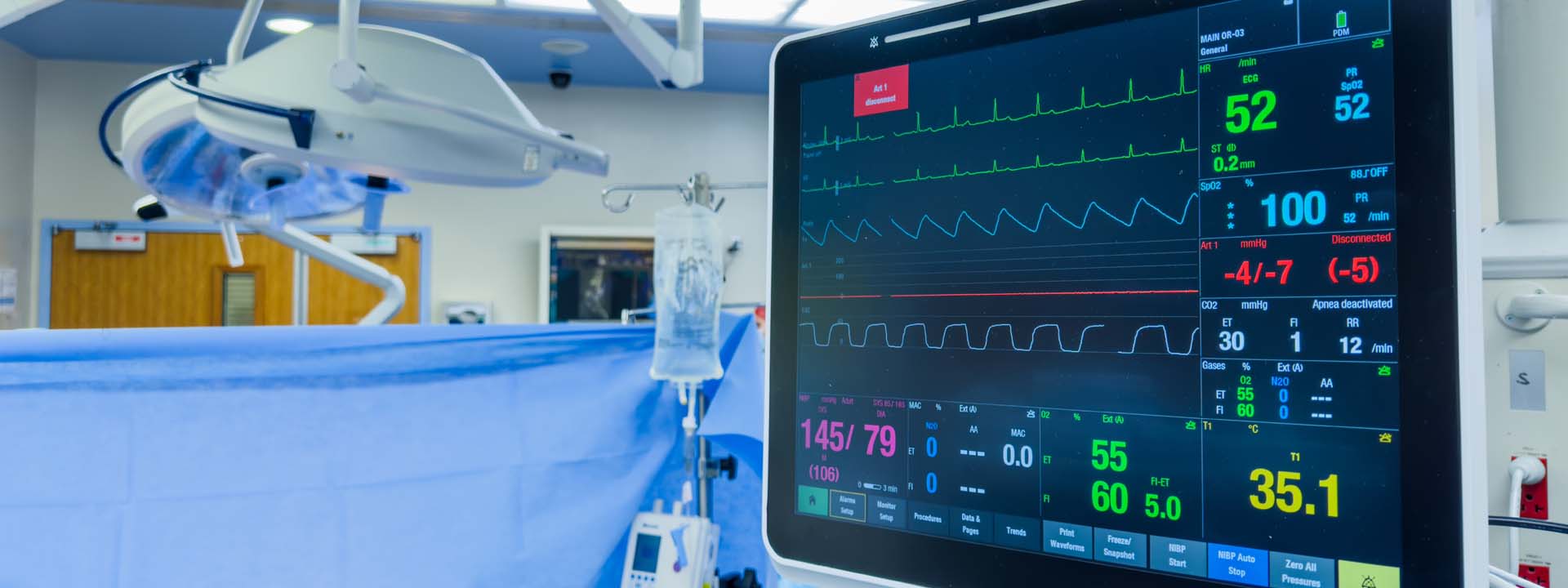
Eurofins Electrical & Electronics offers a range of testing and certification services to help you bring your medical devices to market on time and within budget. Our international network of accredited electrical and electronics laboratories and certification bodies offers a comprehensive range of testing and certification services for active and non-active medical devices and in vitro diagnostics (IVDs).
Our experience and expertise give you the confidence to design, develop, and market tested and certified products efficiently and cost-effectively in your chosen markets.
Our core services include:
- Medical Device Certification
- Notified Body Services for the EU
- Approved Body Services for the UK
- North American Certification Services
- Quality Management Systems & Audits
- Testing of Medical Devices
- Product Safety
- EMC
- Radio/Wireless
- Mechanical & Climatic Simulations
- Performance
- Cybersecurity
Please note that the services of the EU Notified Body and the UK Approved Body are provided through our network of Eurofins Electrical & Electronics laboratories.
For further information, please contact us or view the available services for medical device certification.
Meet the demands of global markets
The market for medical devices is global, and regulatory requirements apply in all major markets to ensure that products meet the highest standards and do not pose a risk to patients or users.
These regulatory requirements and approval procedures vary from country to country and are rarely mutually recognized.
For example, products must comply with EU regulations in the European Economic Area, MHRA requirements in the United Kingdom, FDA requirements in the United States, Health Canada requirements in Canada, JPAL regulations in Japan, and TGA requirements in Australia.
In addition to these markets, many major countries worldwide have their own regulatory systems for medical devices, and managing these requirements is complex and time-consuming. We offer you a single point of contact for the testing and certification of your medical devices, giving you fast, easy, and cost-effective access to the markets of your choice.
Contact our team of experts.
Contact our experts today to begin testing and certifying your products. We offer comprehensive testing, certification, and quality assurance services for electrical and electronic products, simplifying global regulatory compliance and accelerating your time to market.